You'll receive the latest updates on new standards, guidelines, and educational resources, as well as expert insights to help enhance your laboratory's performance and compliance.
Digging Deeper into Understanding Cefazolin Reporting for Enterobacteriaceae
Written By: Audrey N. Schuetz, Mayo Clinic, Rochester, MN and Stella Antonara, Nationwide Children’s Hospital, Columbus, OH
In the Spring 2018 CLSI AST News Update, we provided a case study illustrating various ways by which to report cefazolin as a surrogate for oral cephalosporins for applicable Gram-negative organisms isolated from the urine. The cases concentrated solely on testing and reporting of cefazolin as a surrogate. However, cefazolin, which is administered intramuscularly (IM) or intravenously (IV) may also be used itself for the treatment of complicated urinary tract infections. Additionally, cefazolin may be used in treatment of systemic infections due to susceptible isolates of Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis.
Here, we present two cases that relate to using parenteral cefazolin as the therapeutic agent and one additional case of use of oral cephalosporins for uncomplicated urinary tract infections, as follows:
Case 1 - Reporting of cefazolin for complicated urinary tract infections (cUTIs) when a parenteral agent is indicated
Case 2 - Reporting of cefazolin for treatment of systemic infections due to a susceptible isolate of Escherichia coli
Case 3 - Testing and reporting of the oral agent cefdinir when requested on urine isolates of Citrobacter koseri
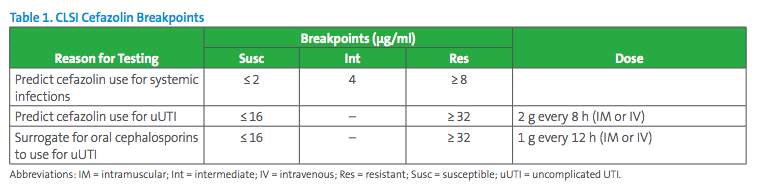
We also provide additional Q&As. The cefazolin breakpoints recommended for use in various settings as listed in CLSI M100-Ed29 are listed in Table 1.
Case Study 1:
A 35-year-old female presents to the Emergency Department (ED) with dysuria and hematuria and lower back pain. The patient is suspected of suffering from acute pyelonephritis which is supported by her clinical symptoms and urinalysis results. Due to severity of symptoms, she is admitted to the hospital. A urine culture is collected and demonstrates >100,000CFU/mL E. coli. The cefazolin MIC is 8µg/mL. Which breakpoints should be applied to cefazolin in this situation?
Answer to Case Study 1:
Due to the status of this patient (acute pyelonephritis), this is considered a case of complicated UTI.1 According to CLSI M100-Ed29, cefazolin MIC breakpoints are S ≤2µg/mL; I 4µg/mL; and R≥8µg/mL when cefazolin is considered for therapy of infections other than uncomplicated UTIs due to E. coli, K. pneumoniae, and P. mirabilis. Rarely do laboratories receive information from clinicians about whether a UTI is considered uncomplicated or not. Sometimes, it can be unclear to the clinicians themselves whether a UTI qualifies as complicated or not. Therefore, laboratories should offer reporting options that can guide clinicians as they narrow or broaden their differentials. The laboratory report may appear as below in Table 2.
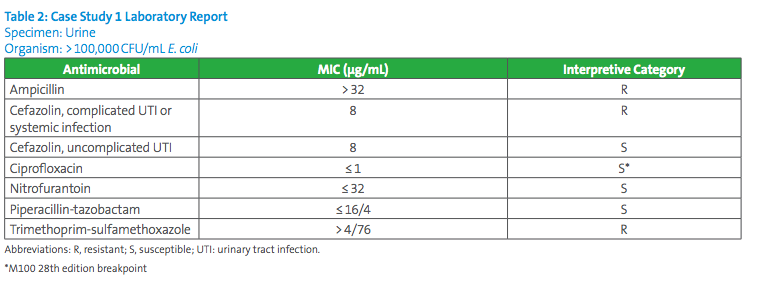
In conclusion, the clinician will likely select another antimicrobial to which the organism tests susceptible since a cefazolin MIC of 8µg/ml is interpreted as resistant for a complicated UTI.
Case Study 2:
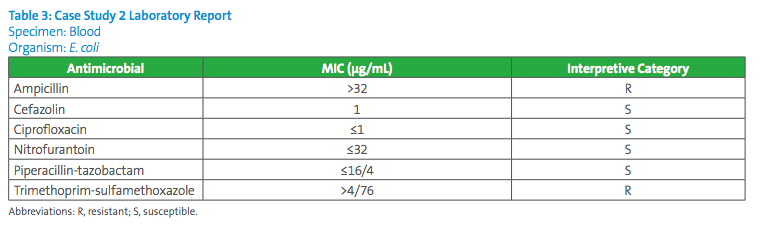
A 50-year-old male presents to the ED with an abscess in his thigh, fever, and urinary symptoms. He has been living on the streets. Methicillin-susceptible Staphylococcus aureus grows from the abscess, and E. coli grows from urine and blood cultures. He was admitted as an inpatient. The clinician had empirically treated with vancomycin and piperacillin-tazobactam. When the culture results were provided, the clinician expressed interest in de-escalating therapy by covering for both S. aureus and E. coli with cefazolin. Ceftriaxone cannot be given due to hyperbilirubinemia in this patient. Which breakpoints should be applied to cefazolin in this scenario?
Answer to Case Study 2:
The cefazolin systemic MIC breakpoints for isolates of Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis are S≤2µg/mL; I 4µg/mL; and R≥8µg/mL. These breakpoints are to be applied for these isolates causing infections other than uUTIs. The clinician would be able to use cefazolin for treatment of this patient’s systemic infection due to E. coli, while covering for confirmed methicillin-susceptible S. aureus. In some laboratories using commercial systems, the lowest cefazolin concentration included in panels is 4µg/mL. If the commercial system does not extend below 4µg/mL and the MIC reported by the instrument is ≤4µg/ml, the isolate could either be intermediate (if the MIC is truly 4µg/mL) or susceptible (if the MIC is truly 2µg/mL or less). The laboratory would need to test the isolate with cefazolin by an alternate method, in order to cover the dilutions below 4µg/mL and report the interpretation of the result based on the systemic breakpoints.
Case Study 3:
A 32-year-old female presents to the ED with dysuria and hematuria. The patient is diagnosed with urinary tract infection which is supported by urinalysis results. A urine culture is collected. The patient is sent home from the ED on cefdinir. The urine culture demonstrates >100,000CFU/mL Citrobacter koseri. Antimicrobial susceptibility testing (AST) results are shown in Table 4. The provider asks the laboratory if cefazolin predicts cefdinir results. How can the laboratory address this request?
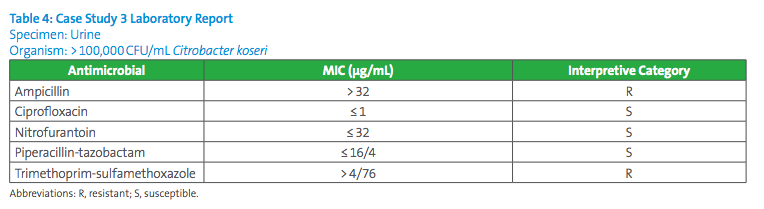
Answer to Case Study 3:
This is a case of uncomplicated UTI in a female patient. According to CLSI, use of cefazolin as a surrogate for oral cephalosporins only applies to E. coli, K. pneumoniae, and P. mirabilis. When the cefazolin surrogate studies were performed, there were only enough data from isolates of these three species to establish surrogacy with confidence. Thus, the laboratory technologist should tell the clinician that cefazolin results cannot predict results for oral cephalosporin agents such as cefdinir. The provider could communicate with the patient and ask if her condition is improving. If she is not improving, the provider can switch the patient to another antibiotic such as nitrofurantoin since it tested susceptible. If the provider insists on testing the isolate for cefdinir susceptibilities, the laboratory could test the C. koseri isolate using an MIC method. Breakpoints for cefdinir exist for the Enterobacteriaceae family. However, the laboratory should be aware that disk diffusion testing for cefdinir and loracarbef should not be performed for Citrobacter, Providencia, or Enterobacter spp., as false-susceptible results have been reported.
Supplemental Q&A:
Question #1
A laboratory is in the process of performing a validation study for the current CLSI cefazolin breakpoints. The lowest concentration of cefazolin on the commercial antimicrobial susceptibility test panel is 4 µg/ml. How can the laboratory validate the cefazolin systemic breakpoints?
Answer #1
If the lowest concentration is 4 µg/ml, the laboratory cannot validate or apply the systemic breakpoints. The laboratory should consult with the Antibiotic Stewardship Team as to how often providers may need cefazolin results for systemic infections and explain the current testing situation. Although there are breakpoints for cefazolin against E. coli, K. pneumoniae, and P. mirabilis, wide use of cefazolin for treatment of non-urinary tract infections due to these species is unlikely. If cefazolin were specifically requested on a non-urine isolate, the laboratory could perform another method of AST (eg, disk diffusion). Nevertheless, the laboratory can still validate the urine breakpoints.
Question #2
Is it likely that manufacturers of commercial antimicrobial susceptibility test systems will obtain FDA clearance for the current CLSI systemic and urine cefazolin breakpoints on their systems in the near future?
Answer #2
No. The FDA cefazolin MIC breakpoints are currently S ≤1 µg/ml, I 2 µg/ml, R ≥ 4 µg/ml and there is no distinction for systemic versus urine breakpoints. Manufacturers of commercial antimicrobial susceptibility test systems must use FDA breakpoints. Consequently, it is unlikely that such systems will be updated with the current CLSI cefazolin breakpoints any time soon.
Reference:
1Gupta K, Hooton TM, Naber KG, et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103–e120.
This article came from AST News Update, Volume 4, Issue 1 – January 2019 which is produced by the CLSI Outreach Working Group (ORWG). The ORWG is part of the CLSI Subcommittee on Antimicrobial Susceptibility Testing (AST) and was established in 2015. The formation of the working group originated in a desire to efficiently convey information regarding contemporary AST practices, recommendations, and resources to the clinical microbiology community. They welcome suggestions from you about any aspect of CLSI documents, educational materials, or their Newsletters.