You'll receive the latest updates on new standards, guidelines, and educational resources, as well as expert insights to help enhance your laboratory's performance and compliance.
Differences in Disk Content Recommended by CLSI and EUCAST for Disk Diffusion Testing
Written By: Romney M. Humphries, Accelerate Diagnostics, Tucson, AZ
Disk diffusion testing is used by a variety of laboratories throughout the world. Disk diffusion breakpoints are dependent on the concentration of antimicrobial impregnated into the disk, otherwise known as the disk content. This disk content in microgram concentrations is printed on each disk and is listed in both CLSI’s M100 and M45 and the European Committee on Antimicrobial Susceptibility Testing (EUCAST)'s breakpoint tables.
Drug manufacturers and standards organizations attempt to identify a disk content that clearly differentiates a susceptible from a resistant isolate. Additionally, attempts are made to ensure the disk content selected does not produce exceedingly large zones for susceptible isolates. For some antimicrobials, no disk content produces acceptable results and consequently disk diffusion testing is not recommended for the drug or drug/organism combination (eg, vancomycin for staphylococci).
There are some differences between CLSI and EUCAST recommended disk contents and not all of these types of disks are commercially available in all countries (Table 1). If a laboratory chooses to apply EUCAST breakpoints to tests performed in the US, it is imperative that the FDA-cleared disk used contains the disk content assigned by EUCAST, and vice versa. Similarly, the disk quality control ranges are based on the disk content, and laboratories must ensure they are using the correct quality control range for the disk content employed.
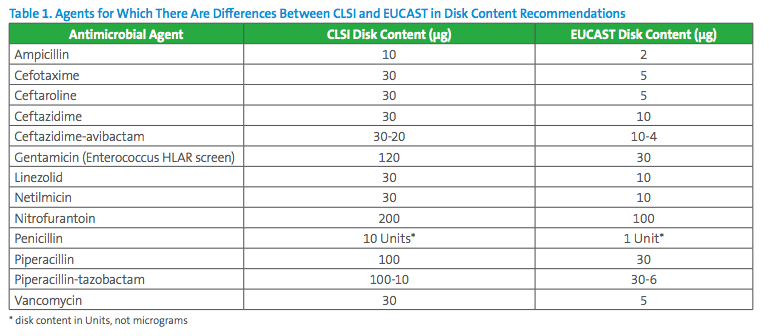
In 2017, CLSI and EUCAST formed a joint disk-diffusion working group endeavored to harmonize disk contents for new antimicrobial agents going forward.
This article came from AST News Update, Volume 4, Issue 1 – January 2019 which is produced by the CLSI Outreach Working Group (ORWG). The ORWG is part of the CLSI Subcommittee on Antimicrobial Susceptibility Testing (AST) and was established in 2015. The formation of the working group originated in a desire to efficiently convey information regarding contemporary AST practices, recommendations, and resources to the clinical microbiology community. They welcome suggestions from you about any aspect of CLSI documents, educational materials, or their Newsletters.