You'll receive the latest updates on new standards, guidelines, and educational resources, as well as expert insights to help enhance your laboratory's performance and compliance.
AST News Update June 2023: New CLSI Intrinsic Resistance Guidance for Fungi

Audrey N. Schuetz, Mayo Clinic, Rochester, MN
Case:
A 60-year-old female with a history of colon cancer presented to the Emergency Department with acute onset of abdominal pain. She had a recent proctocolectomy (removal of rectum and part of colon). Her temperature was normal, blood pressure was 136/70, and heart rate was 149 beats per minute.
Her abdomen was tender throughout. A pelvic abscess was seen on abdominal imaging. Draining of the abscess produced dark debris that looked like stool, suggestive of a bowel leak. The patient’s peripheral white blood cell count was elevated at 10.7 x 109 cells/L (normal range 3.4 – 9.6 x 109 cells/L).
Gram stain of the pelvic fluid showed:
• Few white blood cells.
• Few gram-positive cocci.
• Few gram-positive bacilli.
• Few yeast.
The patient was started empirically on cefepime, metronidazole, vancomycin, and voriconazole to cover for aerobes, anaerobes, and yeasts. Aerobic bacterial and fungal cultures of the abscess were positive for multiple organisms: Enterococcus hirae, Lacticaseibacillus (Lactobacillus) rhamnosus, and Candida krusei (Pichia kudriavzevii). Anaerobic bacterial culture was not ordered. Blood cultures were negative. The E. hirae was susceptible to daptomycin and vancomycin, but susceptibility testing was not performed on L. rhamnosus. Antifungal susceptibility testing (AFST) was performed on C. krusei since yeasts were present on the direct Gram stain and due to the patient’s complicated medical course. Refer to Table 1 for preliminary AFST results for C. krusei performed using a commercial broth microdilution method. Minimal inhibitory concentrations (MICs) were interpreted according to breakpoints and epidemiological cutoff values (ECVs) available in CLSI documents M27M44S and M57S.1,2
Table 1. Preliminary Antifungal Susceptibility Test Results and Report Comment for Candida krusei
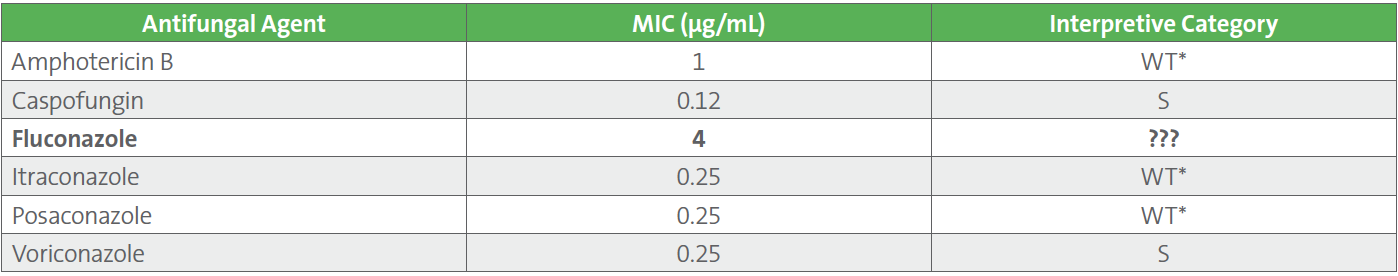
Breakpoints for caspofungin and voriconazole were applied to the organism. ECVs were applied to amphotericin B, itraconazole and posaconazole according to CLSI M57S because breakpoints do not exist.2 There is no breakpoint or ECV for C. krusei and fluconazole.
Should fluconazole be reported? If so, how?
Case Study Answer:
Over the past several years, the Intrinsic Resistance Working Group of the CLSI Subcommittee on Antifungal Susceptibility Tests (AFST SC) has been developing fungal intrinsic resistance (IR) guidance for laboratories. This guidance was developed, in part, due to the microbiology checklist item (MIC.42740) introduced by the College of American Pathologists in 2015 which indicates that unusual or inconsistent antifungal test results should be further investigated by the laboratory. The AFST SC realized at the time that there was limited guidance concerning antifungal test results that are inconsistent with the species identification. Thus, the AFST Intrinsic Resistance Working Group was formed to study IR in both yeasts and molds. IR is defined as inherent or innate (not acquired) antimicrobial resistance which is reflected in wild-type antimicrobial patterns of all or almost all representatives of a species. IR is so common that susceptibility testing is unnecessary. Members of the IR Working Group review population MIC distributions for organisms, gather clinical data on outcomes, and review expert opinion by professional societies on various organism-antifungal combinations. This group’s approach was modeled after that of the IR Working Group of CLSI’s Antimicrobial Susceptibility Testing Subcommittee (AST SC) which investigates bacterial IR.
After formal assessment, CLSI concluded that C. krusei is intrinsically resistant to fluconazole. Many studies with thousands of isolates tested by reference CLSI methodology demonstrate high modal MICs of 16 μg/mL or greater for C. krusei against fluconazole. Professional organizations such as the Infectious Diseases Society of America also recommend against use of fluconazole to treat infections due to C. krusei based on poor clinical response.
In this Case Study example, fluconazole should be reported as resistant, despite the relatively low MIC obtained. This can be achieved by removing the MIC value and reporting a “resistant” categorical result. Table 2 demonstrates the final AFST report for the C. krusei isolate.
Table 2. Final Antifungal Susceptibility Test Results and Report Comment for Candida krusei
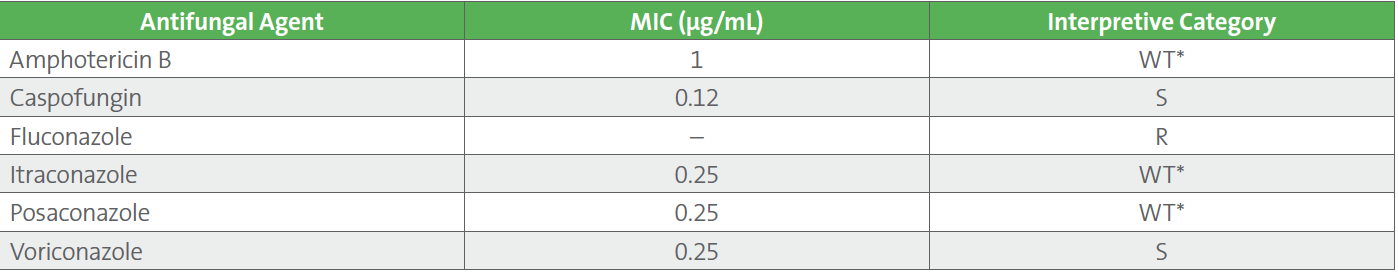
Because antifungal treatment is often empiric, IR comments may be helpful to report before AFST results are available so certain drugs might be avoided. IR comments may be linked with an isolate (rather than with AFST results) so that they are released with the organism name alongside other comments such as “AFST results pending.” A simple report comment, such as “C. krusei is intrinsically resistant to fluconazole,” can be helpful in guiding empiric therapy. Such IR comments are particularly helpful when the fungus must be sent out to a reference laboratory for testing and the turnaround time to results will be prolonged. They can also be helpful even if AFST is not performed on an isolate, in order to lead the clinician away from using a certain antifungal agent.
Reporting decisions for IR should be undertaken by the microbiology laboratory in consultation with the antimicrobial stewardship team and other relevant institutional stakeholders.
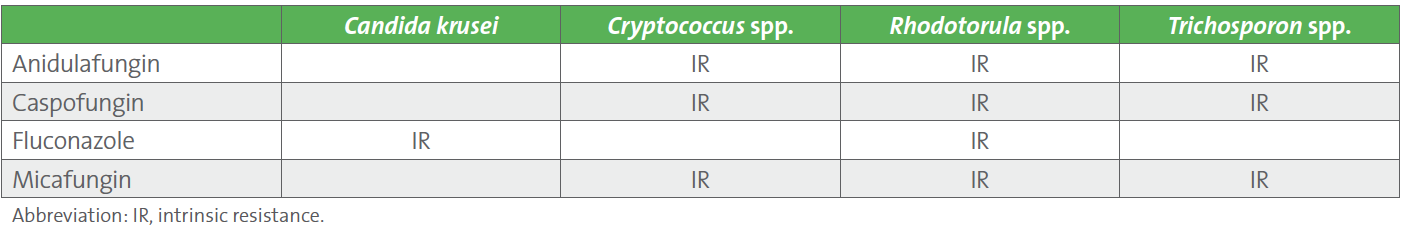
Over 20 fungal-antifungal combinations have been assessed for IR by CLSI, and IR has been determined for several fungi, including yeasts and molds. Intrinsic resistance tables are available in appendixes of the M27M44S (yeast) and M38M51S (mold) documents.1,3 M27M44S is now freely available online. Refer to Table 3 below which was extracted from M27M44S for a list of yeasts which are intrinsically resistant to certain antifungal agents. The M57S document on ECVs for fungi includes a comprehensive summary table outlining available breakpoints, ECVs, and IR for all fungi (yeasts and molds) in Table 6.2
Table 3. Intrinsic Resistance of Yeasts1
Case Follow-up:
The patient’s symptoms resolved with antibiotics and drainage of the abscess. She was discharged home on daptomycin, ertapenem, and voriconazole. The ertapenem was maintained for gram-negative and anaerobic coverage. It is common practice to include such antimicrobials to provide coverage for the variety of bowel microbiota which may be present but may not be recovered in culture.
References
1 CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts. 3rd ed. CLSI supplement M27M44S. Clinical and Laboratory Standards Institute; 2022.
2 CLSI. Epidemiological Cutoff Values for Antifungal Susceptibility Testing. 4th ed. CLSI supplement M57S. Clinical and Laboratory Standards Institute; 2022.
3 CLSI. Performance Standards for Antifungal Susceptibility Testing of Filamentous Fungi. 3rd ed. CLSI supplement M38M51S. Clinical and Laboratory Standards Institute; 2022.
For more articles and issues of the AST News Update, click here.