You'll receive the latest updates on new standards, guidelines, and educational resources, as well as expert insights to help enhance your laboratory's performance and compliance.
AST News Update June 2023: New! CLSI M100-Ed33: Updated Aminoglycoside Breakpoints for Enterobacterales and Pseudomonas aeruginosa

Romney M. Humphries, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Major changes to the aminoglycoside (gentamicin, tobramycin, and amikacin) breakpoints were published in CLSI M100-Ed33 (see Table 1).
Table 1. Status of Breakpoint Revisions for Aminoglycosides in CLSI M100-Ed33
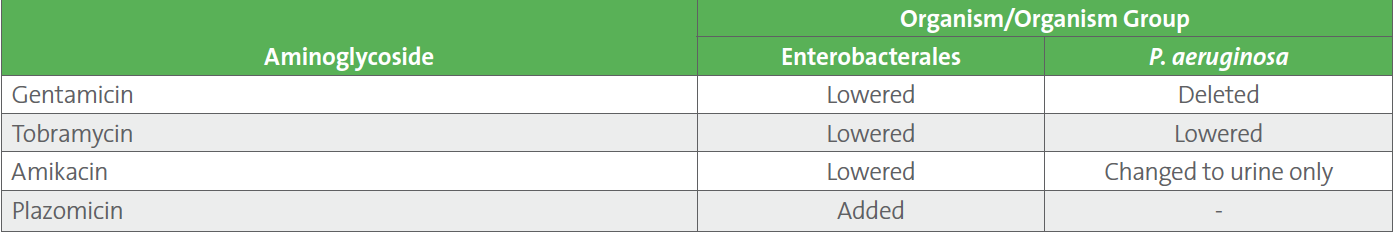
No changes were made to aminoglycoside breakpoints for Acinetobacter spp. or “Other Non-Enterobacterales.”
Background and Reasons for the Changes
Aminoglycosides inhibit protein synthesis by binding to the aminoacyl site of the 16S ribosomal RNA. This antimicrobial class has activity against both gram-positive and gram-negative bacteria, as well as many Mycobacterium spp. and some parasites. Aminoglycosides have no activity against anaerobic bacteria and are inactive against Burkholderia, Stenotrophomonas, Streptococcus, and Enterococcus (with the exception of use in combination therapy to attain synergy for the enterococci). Although aminoglycosides may appear active against Salmonella and Shigella in vitro, they are ineffective against these genera clinically. Today, the most common use of the aminoglycosides is to treat serious infections caused by aerobic gram-negative bacilli, either alone or as part of combination therapy.
Resistance to the aminoglycosides in gram-negative bacteria occurs by three primary pathways:
1. Inactivation of the aminoglycoside by the bacterium’s production of aminoglycoside-modifying enzymes that acetylate, phosphorylate, or adenylate the drugs
2. Alteration of the bacterial ribosomal target site through methylation
3. Decreasing the cell wall permeability to the aminoglycosides, particularly for P. aeruginosa
The newest aminoglycoside, plazomicin (released in 2018), was engineered to overcome the action of aminoglycoside-modifying enzymes, and breakpoints for plazomicin and Enterobacterales only are published for the first time in CLSI M100-Ed33.
Aminoglycoside use is associated with nephrotoxicity and ototoxicity. Nephrotoxicity is mitigated through use of off-label, high-dose extended interval (once-daily) dosing, which is now the standard of care, as opposed to multiple daily dosing.1
The aminoglycoside (gentamicin, tobramycin, and amikacin) breakpoints had not been reexamined since their introduction in the 1980s. However, review of modern pharmacokinetic and pharmacodynamic (PK/PD) data against members of the Enterobacterales and P. aeruginosa demonstrated:
• No safe aminoglycoside dosing regimen was predicted to achieve bacterial 1- or 2-log killing, regardless of the breakpoint applied (2022 or 2023).
• Bacterial stasis (ie, growth inhibition) was achievable with the aminoglycosides using extended interval dosing, but only for isolates with minimal inhibitory concentrations (MIC) below the susceptible breakpoints listed in CLSI M100-Ed32 (see Table 2).
Extensive analysis of PK/PD, clinical outcomes, and current susceptibility profiles for Enterobacterales and P. aeruginosa led CLSI to an update of the aminoglycoside breakpoints (see Table 2).
An important change with the updated breakpoints is the elimination of gentamicin as a suggested treatment option for P. aeruginosa. A maximum gentamicin MIC of 0.5 μg/mL for P. aeruginosa was predicted to achieve the exposure required for bacterial stasis, which is far below the P. aeruginosa epidemiological cutoff value (ECV) of 8 μg/mL. In other words, the data demonstrated wild-type isolates (with MICs below the ECV) were not treatable with gentamicin, which raises the possibility of intrinsic resistance, although intrinsic resistance has not been formally addressed by the Intrinsic Resistance Working Group of the CLSI AST Subcommittee. Increasing the dose of gentamicin is not possible due to the risk of toxicity.
Importantly, the breakpoints in CLSI M100-Ed33 were established using a stasis (rather than a 1- or 2-log kill) endpoint. In other words, isolates that are susceptible by these breakpoints are anticipated to have their growth inhibited, but not be killed, by the aminoglycosides when given as monotherapy. Bacteriostasis endpoints like these are suitable for infections with lower bacterial burden, good source control, and for patients with fewer comorbidities and for whom the consequences of inadequate therapy are low, such as urinary tract infections (UTIs). For this reason, CLSI cautions that monotherapy with the aminoglycosides should be only used for UTIs. Combination therapy for indications other than UTIs should be considered, along with consultation with an infectious diseases specialist.
Important comments related to the updated aminoglycoside breakpoints that appear in CLSI M100-Ed33 are listed in Table 3.
Next Steps
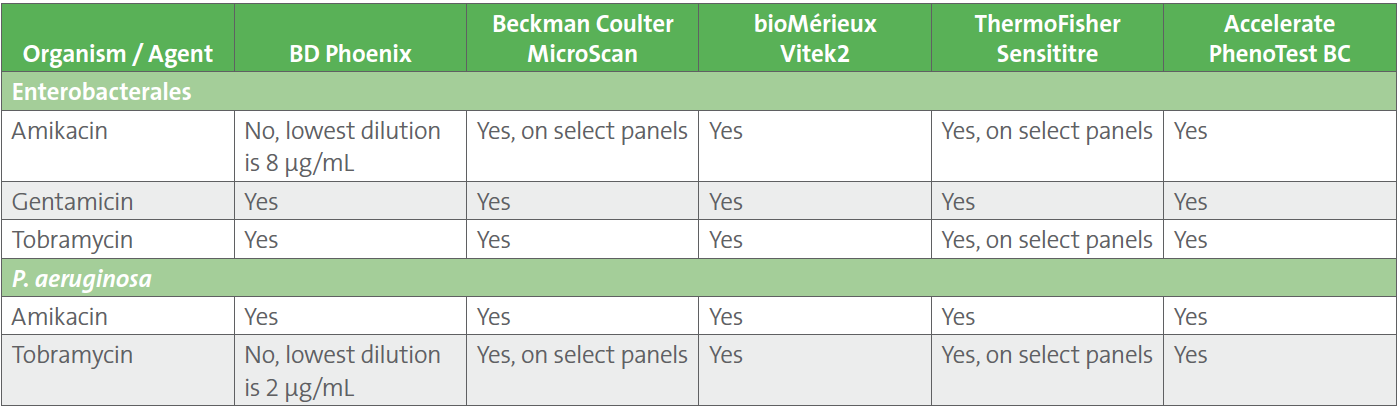
Laboratories should discuss the aminoglycoside breakpoint changes listed in CLSI M100-Ed33 with their institution’s antimicrobial stewardship programs, as well as with infectious diseases clinicians and pharmacy. The US Food and Drug Administration (FDA) has not yet recognized these revised breakpoints, meaning no commercial manufacturer can obtain FDA-clearance for the updated Enterobacterales and P. aeruginosa aminoglycoside breakpoints. Laboratories may consider adoption of the updated breakpoints, off-label, following a validation study, if their test system includes MIC dilutions low enough to accommodate the breakpoints (see Table 4).
Interim steps may include:
1. Enterobacterales and P. aeruginosa:
• Add comment when aminoglycosides are reported.
Example:
“Aminoglycosides should not be used as monotherapy for systemic infections. Consultation with an infectious diseases specialist is recommended.”
• Suppress aminoglycosides and report on request only, using disk diffusion validated using the 2023 breakpoints.
• Implement new aminoglycoside breakpoints on commercial AST system, if possible .
2. P. aeruginosa:
• Suppress or report gentamicin as “R”.
• Report amikacin only on urine isolates.
Table 2. Enterobacterales and P. aeruginosa MIC Breakpoints (μg/mL) for Gentamicin, Tobramycin, Amikacin, and Plazomicin 1
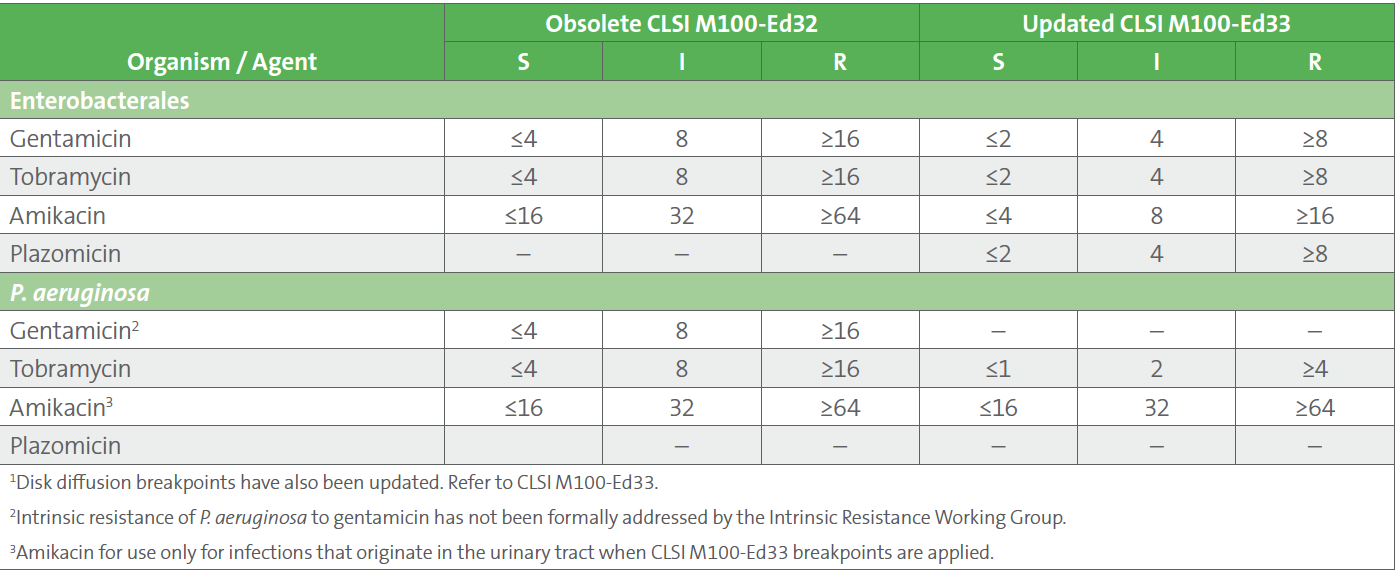
Table 3. New Comments Published in CLSI M100-Ed33 to Accompany the Updated Aminoglycoside Breakpoints

Table 4. Availability of Low Aminoglycoside Dilutions on Automated AST Systems to Accommodate CLSI M100-Ed33 Breakpoints
References
1 Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother. 1995;39(3):650-655. doi: 10.1128/AAC.39.3.650. PMID: 7793867; PMCID: PMC162599.
2 CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 32nd ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2022.
3 CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 33rd ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2023. Access here.
For more articles and issues of the AST News Update, click here.