You'll receive the latest updates on new standards, guidelines, and educational resources, as well as expert insights to help enhance your laboratory's performance and compliance.
Imipenem-Relebactam and Aztreonam-Avibactam: What Do Clinical and Public Health Microbiologists Need to Know?
Paula Snippes Vagnone, Minnesota Department of Health, St. Paul, MN Priyanka Uprety, Robert Wood Johnson Medical School, New Brunswick, NJ Arryn Craney, Weill Cornell Medicine, New York, NY
Novel antimicrobial agents like imipenem-relebactam (IMR), aztreonam-avibactam (AZT-AVI), and cefiderocol have been recently added to the antimicrobial armamentarium to combat multidrug resistant gram-negative infections. Guidelines for clinical microbiology laboratories regarding cefiderocol were addressed in the July 2020 CLSI AST News Update and included questions a laboratory should consider when deciding how to approach testing of these new agents. This current issue provides an update on IMR and AZT-AVI. AZT-AVI will be discussed in terms of in vitro testing and its investigational use for specific multidrug-resistant organisms (MDROs) as it is not currently US Food and Drug Administration (FDA)-approved for clinical use.
Paula Snippes Vagnone, Minnesota Department of Health, St. Paul, MN Priyanka Uprety, Robert Wood Johnson Medical School, New Brunswick, NJ Arryn Craney, Weill Cornell Medicine, New York, NY
Novel antimicrobial agents like imipenem-relebactam (IMR), aztreonam-avibactam (AZT-AVI), and cefiderocol have been recently added to the antimicrobial armamentarium to combat multidrug resistant gram-negative infections. Guidelines for clinical microbiology laboratories regarding cefiderocol were addressed in the July 2020 CLSI AST News Update and included questions a laboratory should consider when deciding how to approach testing of these new agents. This current issue provides an update on IMR and AZT-AVI. AZT-AVI will be discussed in terms of in vitro testing and its investigational use for specific multidrug-resistant organisms (MDROs) as it is not currently US Food and Drug Administration (FDA)-approved for clinical use.
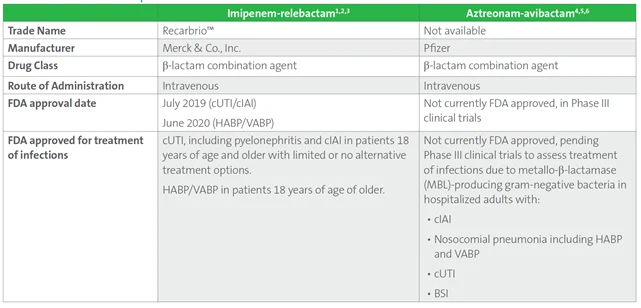
Table 1. Basic Features of Imipenem-relebactam and Aztreonam-avibactam

Table 1. (Continued)


Imipenem-relebactam
1. What is imipenem-relebactam?
Is it like any other antimicrobial agent currently tested? Imipenem-relebactam is a combination of imipenem, the renal dehydropeptidase-1 inhibitor cilastatin and the novel β-lactamase inhibitor relebactam.2, 3
Imipenem binds to penicillin-binding proteins (PBPs) thereby disrupting bacterial cell-wall synthesis. Imipenem activity is low for Proteus, Providencia, and Morganella spp., which is related to poor permeabiltiy and not the presence of a β-lactamase. Cilastatin has no antibacterial activity and is coadministered with imipenem to prevent renal metabolism of imipenem. Relebactam is a diazabicyclooctane class β-lactamase inhibitor that inhibits Class A β-lactamases (CTX-M, TEM, SHV, KPC) and Class C β-lactamases (AmpC). It does not inhibit Class D β-lactamases (OXA-48-like) or Class B β-lactamases (MBLs, VIM, IMP, NDM).7
2. Should imipenem-relebactam be tested routinely?
When might a laboratory be asked to test imipenem-relebactam? According to the most recent Infectious Diseases Society of America (IDSA) Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections, new β-lactam-combination agents like ceftazidime-avibactam (CZA), meropenem-vaborbactam and IMR are the preferred treatment options for carbapenem-resistant Enterobacterales (CRE) infections when additional information on carbapenemase phenotypic/genotypic profile is not readily available.8 Imipenem-relebactam is not recommended for the treatment of CRE infections due to MBL (Class B)- or OXA-48-like β-lactamase (Class D)-producers or members of the Morganellaceae group (i.e. Proteus, Providencia, and Morganella).
The IDSA also recommends consideration of IMR as a preferred treatment option for difficult-to-treat P. aeruginosa infections (defined as not susceptible to piperacillin-tazobactam, ceftazidime, cefepime, aztreonam, meropenem, imipenem, ciprofloxacin, and levofloxacin). Other first-line agents for difficult to treat P. aeruginosa include CZA or ceftolozane-tazobactam, for infections outside the urinary tract. Cefiderocol and single-dose aminoglycoside are also first-line options for urinary tract infections. Following discussion with the antimicrobial stewardship team, laboratories may elect to test IMR routinely, by special request or by developing a reflex algorithm for specific carbapenem-resistant gram-negative bacteria.
3. How should imipenem-relebactam be tested (Table 2)? Are there any unique testing considerations?
Routine CLSI reference disk diffusion (aerobes only) and broth microdilution MIC methods can be used for testing IMR.9,10,11 FDA-cleared (as of the date of this publication) commercial systems for IMR, including disks, are listed in Table 2. Check with manufacturer for specific FDA-cleared applications.
Table 2. Testing Options for Imipenem-relebactam and Aztreonam-avibactam

4. How should imipenem-relebactam results be interpreted?
The clinical breakpoints for IMR provided to date by FDA, CLSI and EUCAST are listed in Table 3.
5. What are expected AST results for imipenem-relebactam?
According to the SMART, surveillance program Enterobacterales isolates tested from 2015-2018 show good overall performance for IMR with ~95% of all isolates testing susceptible. Relebactam restored susceptibility to imipenem at the following percentages when testing imipenem-not susceptible isolates of: E. coli (48.8%), K. pneumoniae (74.9%), E. cloacae (46.1%), K. aerogenes (90.8%), K. oxytoca (37.5%), and C. freundii (65.2%).12
IMR does not offer additional advantage for isolates that are resistant to imipenem by mechanisms other than Class A and C β-lactamases. Since Morganella spp., Proteus spp., and Providencia spp. demonstrate elevated MICs to imipenem due to a β-lactamase independent mechanism, IMR is not useful for these species.
The activity of IMR on carbapenem-resistant P. aeruginosa (n=1,445) depends on the individual carbapenemase gene present. Findings from a recent study demonstrated 97.3% of isolates tested susceptible to IMR compared to 94.6% and 94.2% for ceftolozane-tazobactam and CZA, respectively.13 Imipenem-relebactam offers limited benefit for Acinetobacter baumannii infections due to the presence of Class D β-lactamases commonly found in this species and has no activity against S. maltophilia, which is intrinsically resistant to imipenem.
Table 3. FDA, CLSI and EUCAST Breakpoints for Imipenem-relebactam

Aztreonam-avibactam
1. What is aztreonam-avibactam? Is it like any other antimicrobial agent currently tested?
Aztreonam-avibactam (ATM-AVI) is not yet FDA approved, but is pending Phase III clinical trials. Aztreonam is the only clinically available member of the monobactam class of antimicrobial agents and, uniquely, is not hydrolyzed by MBLs. However, aztreonam is hydrolyzed by other β-lactamases such as those in Ambler Class C (AmpC) and Ambler Class A (eg, KPC) that often are often found in isolates of Enterobacterales. Avibactam is a diazabicyclooctane non-β-lactamase β-lactamase inhibitor that has wide-ranging activity against Ambler Class A and C, and some Class D β-lactamases such as OXA-48-like. In vitro studies have demonstrated that avibactam restores the activity of aztreonam (ATM) against Enterobacterales containing Class B MBLs (eg, NDM, VIM, IMP).4,5,6,14
2. Should aztreonam-avibactam be tested routinely? When might a laboratory be asked to test aztreonam-avibactam?
Aztreonam-avibactam is not FDA approved; however, both ATM and ceftazidime-avibactam (CZA) are clinically available. According to the most recent guidelines from IDSA, the combination of CZA with ATM is a preferred treatment option for infections due to MBL-producing CRE (eg, NDM, IMP, VIM).8
Testing for ATM and CZA individually may not provide adequate information to suggest clinical treatment with ATM-AVI. However, CDC’s Antibiotic Resistance (AR) Lab Network offers testing of patient isolates for ATM-AVI by broth microdilution. The panel includes ATM, CZA, and ATM-AVI, and can be performed on confirmed isolates of MBL-producing Enterobacterales at no charge. MICs and interpretations based on current CLSI M100 breakpoints are reported for ATM and CZA. Since there are no established breakpoints for ATM-AVI, MIC results are reported without interpretation. See the CDC Expanded Antimicrobial Susceptibility Testing for Hard-to-Treat Infections (ExAST) website for details regarding isolate submission criteria, available testing locations, and additional information.
3. What are expected AST results for aztreonam-avibactam?
Although breakpoints are not yet established for ATM-AVI, several in vitro studies of MBL-producing Enterobacterales have demonstrated MICs typically ≤2/4 µg/mL, with a range of ≤0.015/4 to 8/4 µg/mL.5 A recent study of NDM-producing Enterobacterales found some E. coli with MICs within a range of ≤0.03/4 to 32/4 µg/mL.15For some isolates of non-MBL-producing Enterobacterales, ATM-AVI MICs were >128/4 µg/mL. These organisms may harbor other mechanisms of resistance, such as CMYtype β-lactamases.5
References
1 US Food and Drug Administration. Imipenem-Relebactam (RECARBRIO). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212819Orig1s000TOC.cfm. Accessed April 7, 2021.
2 US FOOD and Drug Administration. HABP/VABP indication for imipenem-relebactam. https://www.fda.gov/news-events/ press-announcements/fda-approves-antibiotic-treat-hospital-acquired-bacterial-pneumonia-and-ventilator-associated. Accessed April 7, 2021.
3 Merck. Recarbrio – Highlights of prescribing information. https://www.merck.com/product/usa/pi_circulars/r/recarbrio/recarbrio_pi.pdf. Accessed April 7, 2021.
4 Sader HS, Mendes RE, Pfaller MA, et al. Antimicrobial activities of aztreonam-avibactam and comparator agents against contemporary (2016) clinical Enterobacteriaceae isolates. Antimicrob Agents Chemother. 2018;62(1):e01856-17.
5 Karlowsky JA, Kazmierczak KM, de Jonge BLM, et al. In vitro activity of aztreonam-avibactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated by clinical laboratories in 40 countries from 2012 to 2015. Antimicrob Agents Chemother. 2017;61(9):e00472-17.
6 NIH U.S. Efficacy, safety, and tolerability of ATM-AVI in the treatment of serious infection due to MBL-producing gram-negative bacteria. https://clinicaltrials.gov/ct2/show/NCT03580044?term=ATM-AVI&draw=2&rank=1. Accessed April 7, 2021.
7 Yahav D, Giske CG, Grāmatniece A, et al. New β-Lactam-β-lactamase inhibitor combinations. Clin Microbiol Rev. 2020;34(1):e00115-20. Erratum in: Clin Microbiol Rev. 2021;34(2):e00021-21.
8 Tamma PD, Aitken SL, Bonomo RA, et al. Infectious Diseases Society of America guidance on the treatment of extended spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. 2020;27:ciaa1478. doi: 10.1093/cid/ciaa1478.
9 CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests. 13th ed. CLSI standard M02. Wayne, PA: Clinical and Laboratory Standards Institute; 2020.
10 CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 11th ed. CLSI standard M07. Wayne, PA: Clinical and Laboratory Standards Institute; 2020
11 CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2021.
12 Yang Q, Zhang H, Yu Y, et al. In vitro activity of imipenem/relebactam against Enterobacteriaceae isolates obtained from intra-abdominal, respiratory tract, and urinary tract infections in China: study for monitoring antimicrobial resistance trends (SMART), 2015-2018. Clin Infect Dis. 2020;71(Supplement_4):S427-S435.
13 Fraile-Ribot PA, Zamorano L, Orellana R, et al. GEMARA-SEIMC/REIPI Pseudomonas Study Group. Activity of imipenemrelebactam against a large collection of Pseudomonas aeruginosa clinical isolates and isogenic β-lactam-resistant mutants. Antimicrob Agents Chemother. 2020;64(2):e02165-19.
14 Vasoo S, Cunningham SA, Cole NC, Kohner PC, et al. In vitro activities of ceftazidime-avibactam, aztreonam-avibactam, and a panel of older and contemporary antimicrobial agents against carbapenemase-producing gram-negative bacilli. Antimicrob Agents Chemother. 2015;59(12):1835–1846.
15 Lutgring JD, Balbuena R, Reese N, et al. Antibiotic susceptibility of NDM-producing Enterobacterales collected in the United States in 2017 and 2018. Antimicrob Agents Chemother. 2020; 64(9): e00499-20.
This article came from AST News Update April 2021 which is produced by the CLSI Outreach Working Group (ORWG). T
he ORWG is part of the CLSI Subcommittee on Antimicrobial Susceptibility Testing (AST) and was established in 2015. The formation of the working group originated in a desire to efficiently convey information regarding contemporary AST practices, recommendations, and resources to the clinical microbiology community. They welcome suggestions from you about any aspect of CLSI documents, educational materials, or their Newsletters.