You'll receive the latest updates on new standards, guidelines, and educational resources, as well as expert insights to help enhance your laboratory's performance and compliance.
Implementation of the CLSI Method for Direct Disk Diffusion Testing From Positive Blood Cultures
Audrey N. Schuetz, Mayo Clinic, Rochester, MN
April Bobenchik, Penn State Hershey Medical Center, Hershey, PA
Shelley Campeau, Microbiology Consultant, Tucson, AZ
The CLSI AST Subcommittee has developed and published breakpoints for direct blood culture disk diffusion (DD) testing of several antimicrobial agents for Enterobacterales and Pseudomonas aeruginosa. 1 This project was undertaken to provide antimicrobial susceptibility testing (AST) results earlier than traditional AST methods. Rapidity of susceptibility testing and reporting is particularly important for bloodstream infections. Studies have shown that mortality from sepsis increases every hour that the time to first appropriate antimicrobial is delayed.2 CLSI developed this method to provide laboratories with a direct-from-blood culture testing approach which is both simple to perform and inexpensive.
Audrey N. Schuetz, Mayo Clinic, Rochester, MN
April Bobenchik, Penn State Hershey Medical Center, Hershey, PA
Shelley Campeau, Microbiology Consultant, Tucson, AZ
The CLSI AST Subcommittee has developed and published breakpoints for direct blood culture disk diffusion (DD) testing of several antimicrobial agents for Enterobacterales and Pseudomonas aeruginosa. 1 This project was undertaken to provide antimicrobial susceptibility testing (AST) results earlier than traditional AST methods. Rapidity of susceptibility testing and reporting is particularly important for bloodstream infections. Studies have shown that mortality from sepsis increases every hour that the time to first appropriate antimicrobial is delayed.2 CLSI developed this method to provide laboratories with a direct-from-blood culture testing approach which is both simple to perform and inexpensive.
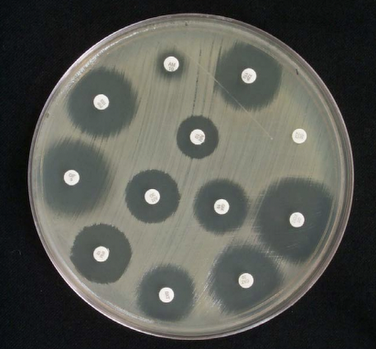
An initial pilot study of direct DD from positive blood cultures demonstrated good performance, and the method has since been optimized.3 To establish breakpoints, results from the direct DD method using positive blood culture broth as the inoculum with incubation of the DD test for 8-10 and 16-18 hours were compared to the standard DD method using isolated colonies with 16-18 hours incubation. For some agents, the standard breakpoints worked, while others needed adjustment. It is important to note that the breakpoints for P. aeruginosa for ciprofloxacin at the 8-10 hour read differ from the standard DD breakpoints for P. aeruginosa for that antimicrobial. Details of this method and DD breakpoints are provided in Tables 3E-1, -2, and -3 of the M100 document.
Recent M100 2022 breakpoint additions for direct blood culture DD include several antimicrobial agents for Enterobacterales and P. aeruginosa, including both early (8-10-hour reading) and overnight (16-18-hour reading) breakpoints for several antimicrobials (see Table 1).
Table 1. CLSI Breakpoints for Direct Blood Culture Disk Diffusion
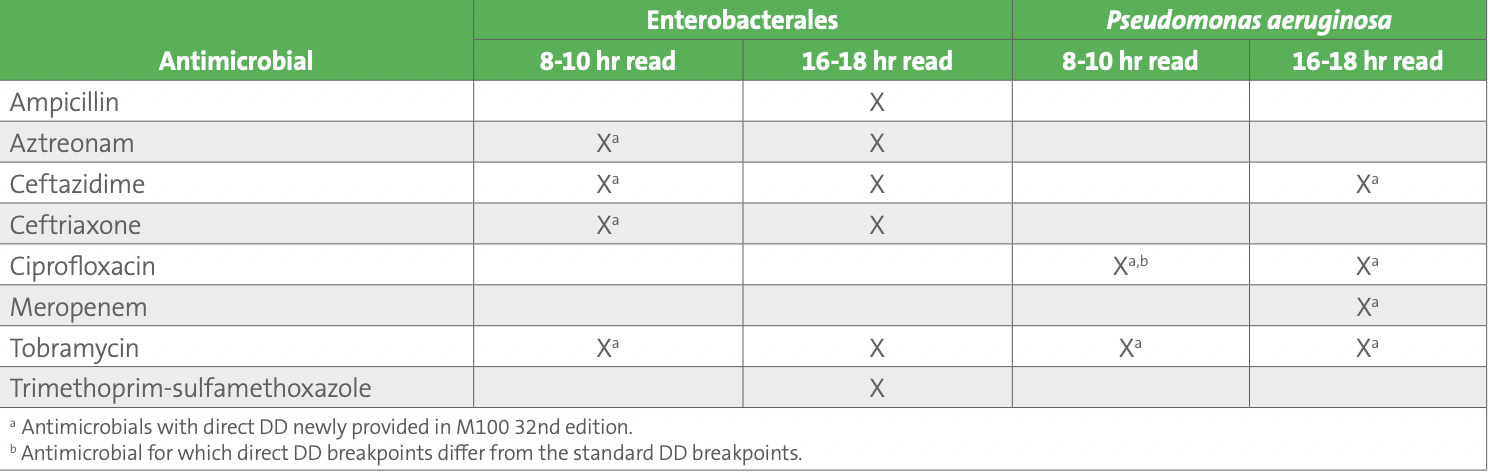
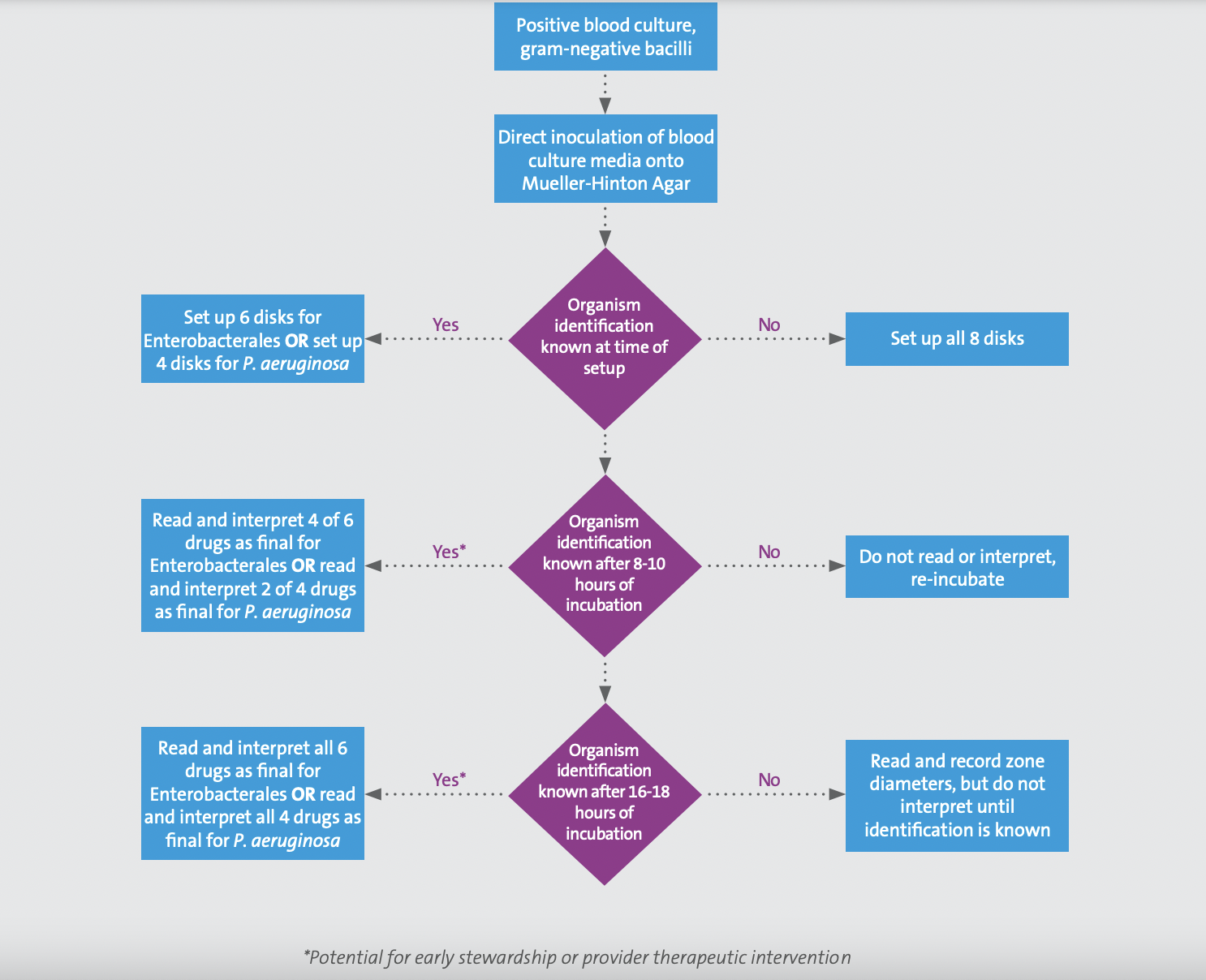
Performance of the direct blood culture DD method must be paired with an identification method since breakpoints are specific to either Enterobacterales or P. aeruginosa. Laboratory workflows for this method vary based on whether or not the organism identification is available at the time of direct DD set up (see Figure 1). It is important to note that direct DD must be set up within 8 hours of the blood culture bottle flagging positive for gram-negative bacilli. If the organism identification is available at the time of direct DD set up, the applicable numbers of disks should be used (ie, if Enterobacterales, set up the six applicable disks for which breakpoints have been developed; if P. aeruginosa, set up the four applicable disks). However, if the identification is other than Enterobacterales or P. aeruginosa, direct DD should not be set up. On the other hand, if identification will be available after direct DD set up, the laboratory should set up all eight disks. Options for identification at the time of direct DD set up include rapid molecular tests or rapid (ie, early) matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS).
Figure 1. Workflow for direct blood culture disk diffusion testing
When reporting and interpreting antimicrobial DD results, follow intrinsic resistance rules provided in Appendix B of M100. Direct DD results are considered final and do not need to be confirmed or repeated; however, routine AST may be necessary to provide additional antimicrobial results. Laboratories should consider who will be notified of the early and overnight direct DD results and develop a notification system with actionable pieces of information regarding direct DD results. Some laboratories may choose to notify the primary patient-facing clinical team and/or they may notify the antimicrobial stewardship team with the direct DD results. CLSI continues to evaluate additional antimicrobials for direct DD method breakpoints for Enterobacterales and P. aeruginosa. Direct DD breakpoints are still under evaluation for piperacillin-tazobactam and cefepime, which typically play a large role in therapy of bloodstream infections. Data on direct DD antimicrobials for Acinetobacter are also being assessed. Publication of the data leading to establishment of these breakpoints is underway, and guidance on initial verification and adoption of this method will also follow in an upcoming CLSI publication (pending). In summary, consider adoption of the direct blood culture DD method regardless of which routine AST system is used in your laboratory. Paired organism identifications are necessary for appropriate result interpretation. As more breakpoints are developed for this method over time, it is important to highlight that those breakpoints listed in Tables 3E of M100 may differ from the standard breakpoints in Tables 2. QC should be performed following routine DD QC protocols.
References
1. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 32nd ed. CLSI supplement M100. Clinical and Laboratory Standards Institute; 2022.
2. Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: Results from a guideline-based performance improvement program. Crit Care Med. 2014:42(8):1749-1755.
3. Chandrasekaran S, Abbott A, Campeau S, et al. Direct-from-blood-culture disk diffusion to determine antimicrobial susceptibility of gram-negative bacteria: Preliminary report from the Clinical and Laboratory Standards Institute Methods Development and Standardization Working Group. J Clin Microbiol. 2018;56(3):e01678-17.