Join our community of industry leaders who volunteer their experience and knowledge to drive innovation and excellence in laboratory practices.
Document Development Committee
What is a Document Development Committee?
Comprised of a balanced representation from government, industry, and the health care professions, a CLSI document development committee is a group of technical experts who work together to develop a CLSI document.
What positions are available and what do they entail?
Comprised of a balanced representation from government, industry, and the health care professions, a CLSI document development committee is a group of technical experts who work together to develop a CLSI document.
*Position has voting rights on the committee.

How often does the committee meet?
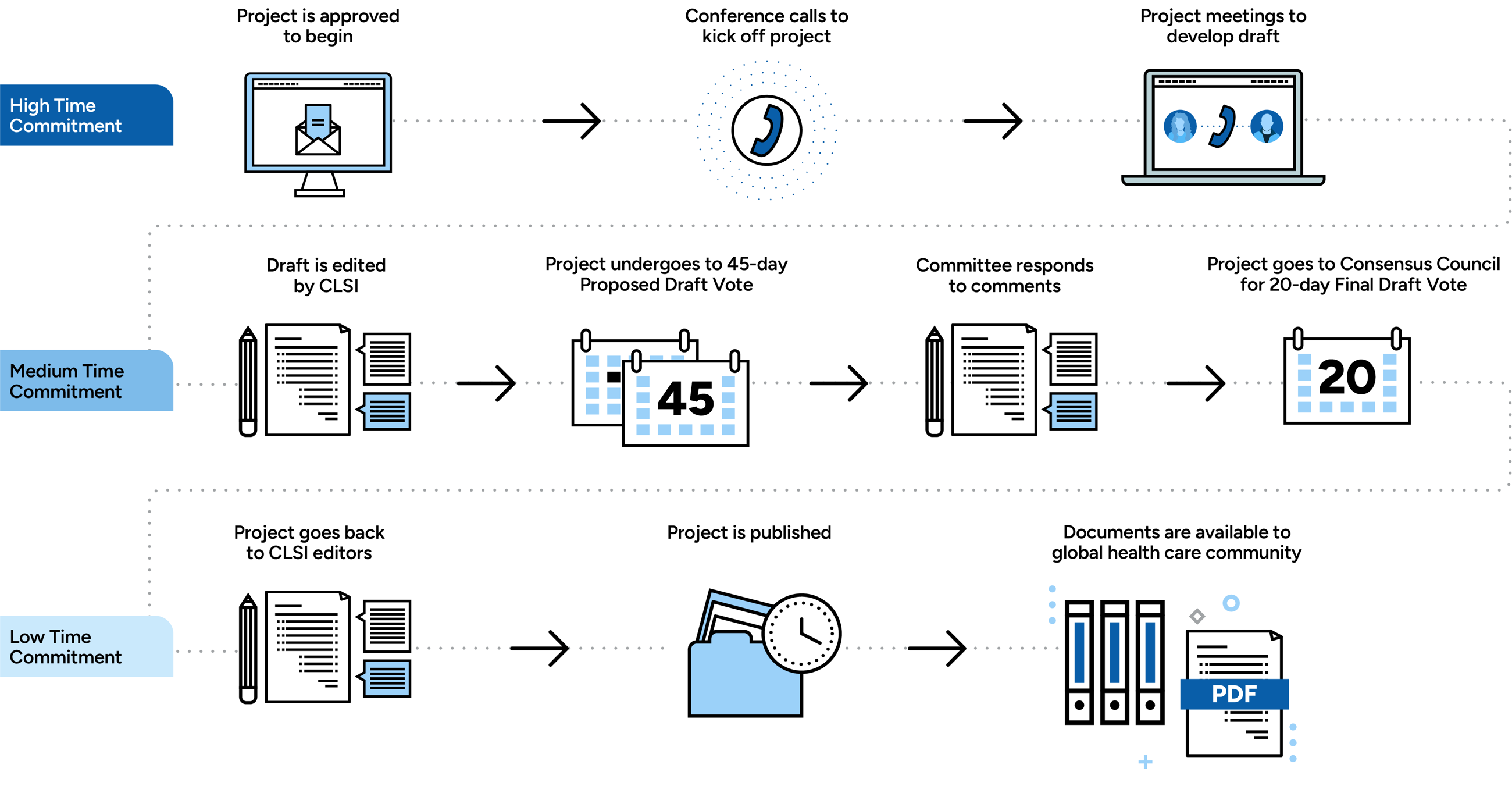
Document development committee volunteers meet virtually several times throughout the project. However, a document development committee typically only meets in person once. There is no attendance fee for meetings, but registration is required. Your CLSI project manager will notify you of the planned and expected meetings at the start of the project, and will provide announcements, agendas, and other pertinent materials before each meeting.
What can I expect as a committee volunteer?
Committee volunteers meet regularly—in person and virtually—throughout the development process to review the draft document, discuss and resolve issues related to the document's content, and to ensure the committee is on schedule with respect to the document development timeline.
Once the draft is finalized by the committee, it is circulated for Proposed Draft review and vote by the committee, the responsible expert panel, and the CLSI member delegates. After the vote, the committee reviews and responds to all comments received. The Final Draft document is then prepared and submitted to the Consensus Council for review and approval. Once approved, the document is prepared for publication.
On average, it takes 24 months to complete a CLSI document. As a committee volunteer, you are asked to commit to participating for the duration of the project.
Document Development Committee Lists - NEW
Our committees are the heart of CLSI. Beginning September 2025, CLSI is publishing DDC lists here on the website. CLSI is grateful for the contributions of all the subject matter experts that generously dedicate their time and expertise to standards development. These laboratorians, clinicians, industry professionals, and academic leaders bring their knowledge and experience to ensure every document reflects best practices and supports quality patient care worldwide. By working together, committee members help drive progress, foster innovation, and uphold the values of excellence in laboratory medicine.
We thank these committed volunteers for their valued contributions and will continue to add new document committee lists as the projects go to public review.
- Kayode Balogun, BSc, MSc, PhD / Contributor / University of Utah Health and ARUP Laboratories, United States, Ended: Sep 3, 2025
- Michelle Brown, MT(AMT) / Contributor / Sentara Healthcare, United States, Ended: Sep 3, 2025
- Jake D. Bunn, MBA, MLS(ASCP)CM / Member / Centers for Disease Control and Prevention, United States, Ended: Sep 3, 2025
- Sheldon M. Campbell, M.D. Ph.D. F.C.A.P. / Member / Yale School of Medicine - for QMS28 CAP, United States, Ended: Sep 3, 2025
- Ruben Cudiamat, MLT(CSMLS) / Contributor / Public Health Ontario Laboratory, Canada, Ended: Sep 3, 2025
- Sabrina DeBose, DHSc, MS, RBP / Member / Centers for Disease Control and Prevention, United States, Ended: Sep 3, 2025
- Cara Faliano, MLS(ASCP), QLS, CQIA, CSSGB(ASQ) / Member / UCHealth University of Colorado Hospital, United States, Ended: Sep 3, 2025
- Matthew Gallman, MS, ASP, QLScm / Member / Oklahoma State Department of Health Public Health Laboratory, United States, Ended: Sep 3, 2025
- Linda Gylland, MLS(ASCP)cm, QLS cm / Contributor / Sanford Health System, United States, Ended: Sep 3, 2025
- Sean G. Kaufman, MPH, CHES, CPH, IFBA, CP / Member / Safer Behaviors, United States, Ended: Sep 3, 2025
- Angela Lee, BSc, MLT, MBA, CQA(ASQ)CMQ/OE / Member / Diagnostic Accreditation Program, Canada, Ended: Sep 3, 2025
- Abiola Lyons, MHA / Contributor / North Central Regional Health Authority, Republic of Trinidad and Tobago, Trinidad And Tobago, Ended: Sep 3, 2025
- Clarah Mutandiro, MT(ASCP)cm / Contributor / Texas Children's Hospital, Ended: Sep 3, 2025
- Adrienne Orach / Contributor / St. Luke's Bethlehem Campus, United States, Ended: Sep 3, 2025
- Michael A. Pentella, PhD, D(ABMM), SM(ASCP), CIC / Contributor / University of Iowa, United States, Ended: Sep 3, 2025
- José Poloni / Contributor / Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Brazil, Ended: Sep 3, 2025
- Tammy Porter, MLS(ASCP) / Member / Ochsner Medical Center Laboratory, United States, Ended: Sep 3, 2025
- Crystal J. Sands, MBA, MT(ASCP)SM, DLMcm, SLScm / Contributor / NorDx - Scarborough Campus, United States, Ended: Sep 3, 2025
- E. Jayne Scoggin, BA, CT, CG, MB(ASCP), CQA(ASQ) / Vice-Chairholder / ResearchDX, United States, Ended: Sep 3, 2025
- Daniel J. Scungio, MT(ASCP), SLS, CQA(ASQ) / Chairholder / Sentara Healthcare, United States, Ended: Sep 3, 2025
- Edgar Enrique Sevilla-Reyes / Contributor, Mexico, Ended: Sep 3, 2025
- Jean Tenuta, MBA, MS, MLS(ASCP)CM, DLM, CQA(ASQ)CMQ/OE / Member / Northwestern Medicine, United States, Ended: Sep 3, 2025
- Ivana Baršić Lapić, MSc / Contributor / University Hospital Centre Zagreb, Croatia, Ended: Sep 25, 2025
- Christina Bethoney, BS / Member / Siemens Healthineers, United States, Ended: Sep 25, 2025
- Jake D. Bunn, MBA, MLS(ASCP)CM / Member / Centers for Disease Control and Prevention, United States, Ended: Sep 25, 2025
- Andrea Caballero Garralda, PhD, MBA / Contributor / European Federation of Clinical Chemistry and Laboratory Medicine, Spain, Ended: Sep 25, 2025
- Douglas W. Chesher, MBBS, PhD, MAACB, FRCPA / Contributor / NSW Health Pathology, Australia, Ended: Sep 25, 2025
- Karl De Vore, BA, SSBB / Chairholder / Bio-Rad Laboratories, Inc., United States, Ended: Sep 25, 2025
- Daniel Figdore, MLS (ASCP) / Member / Mayo Clinic, United States, Ended: Sep 25, 2025
- Ashley Frost, MHA, MLS(ASCP)CM / Vice-Chairholder / Kodiak Area Native Association, United States, Ended: Sep 25, 2025
- Mukesh Gandhi / Contributor / Dynacare (Brampton), Canada, Ended: Sep 25, 2025
- Steven C. Kazmierczak, PhD, DABCC, FACB / Member / Oregon Health and Science University, United States, Ended: Sep 25, 2025
- Mark D. Kellogg, PhD, MT(ASCP), DABCC / Member / Boston Children's Hospital, United States, Ended: Sep 25, 2025
- Fred Leland McClure, MSci, PhD, F-ABFT / Contributor / DTC, United States, Ended: Sep 25, 2025
- Claire Moyer, MBA, MLS(ASCP)CM / Contributor / Labcorp, United States, Ended: Sep 25, 2025
- Jamie Platt, PhD / Member / LabCorp, United States, Ended: Sep 25, 2025
- Donna Roberts, MS, MT(ASCP) / Member / Baylor Scott and White Health, United States, Ended: Sep 25, 2025
- Darika Siegel, MBA, MLS(ASCP) / Contributor / Abbott, United States, Ended: Sep 25, 2025
- Kenneth A. Sikaris, MD / Member / Sonic Healthcare Limited – AUSTRALIA, Australia, Ended: Sep 25, 2025
- Jeannie M. Stubblefield, PhD, NRCC, FADLM / Member / University of Oklahoma Health Science Center, United States, Ended: Sep 25, 2025
- Richard Y. Wang, DO / Contributor / Centers for Disease Control and Prevention, United States, Ended: Sep 25, 2025
- Ruhan Wei, PhD, DABCC / Contributor / DUHS Clinical Laboratories, United States, Ended: Sep 25, 2025
- Kevin Alby, PhD, D(ABMM) / Chairholder / Brown University Health, United States, Ended: Dec 9, 2025
- Esther Babady, PhD, D(ABMM), FIDSA, F(AAM) / Member / bioMérieux, United States, Ended: Dec 9, 2025
- Natali Baker, MS, MLS(ASCP), SM / Contributor / ARUP Laboratories, United States, Ended: Dec 9, 2025
- Sarah Becket, MLS(ASCP)CM / Member / BD - Sparks, MD, United States, Ended: Dec 9, 2025
- Lori Bourassa, PhD, MPH, D(ABMM) / Contributor / University of Washington Medical Center Department of Laboratory Medicine, Ended: Dec 9, 2025
- Kendall Bryant, PhD, D(ABMM) / Contributor / Vanderbilt University Medical Center, United States, Ended: Dec 9, 2025
- Carey-Ann Burnham, PhD, D(ABMM) / Member / Pattern Bioscience, United States, Ended: Nov 15, 2021
- Melanie Carr, MLS(ASCP) / Contributor / BD - Durham, NC, United States, Ended: Dec 9, 2025
- Patricia Cernoch, MT(ASCP)SM, SV / Contributor / Methodist Hospital, United States, Ended: Dec 9, 2025
- Courtney Chandler, PhD / Member / FDA, United States, Ended: Dec 9, 2025
- Melvili Cintron Cotto, PhD / Contributor / Memorial Sloan Kettering Cancer Center, United States, Ended: Dec 9, 2025
- Joshua Cohen / Contributor / Centers for Medicare & Medicaid Services, United States, Ended: Dec 9, 2025
- Karissa Culbreath, PhD, D(ABMM) / Vice-Chairholder / TriCore Reference Laboratories, United States, Ended: Dec 9, 2025
- Jane Drury, B.Sc / Contributor / NSW Health Pathology, Australia, Ended: Jun 28, 2021
- Mark A. Fisher, PhD, D(ABMM) / Member / ARUP Laboratories, United States, Ended: Dec 9, 2025
- Steven Giglio, PhD / Member / LBT Innovations, Australia, Ended: Dec 9, 2025
- Tobin Hellyer, PhD / Member / Retired, United States, Ended: Mar 11, 2024
- S. Wesley Long, MD, PhD, D(ABMM) / Member / Houston Methodist St John Hospital, United States, Ended: Dec 9, 2025
- Erin McElvania, PhD, D(ABMM) / Member / Evanston Hospital, NorthShore University HealthSystem, United States, Ended: Dec 9, 2025
- Jose Luis Moreno, QFB, CLM (ASCPi) / Contributor / Salud Digna A.C., Mexico, Ended: Dec 9, 2025
- Sunday Ogunkola, MSQA, M(ASCP), CQA, ASQ / Member / Centers for Medicare & Medicaid Services, United States, Ended: Dec 9, 2025
- Susan Sharp, PhD, D(ABMM), F(AAM) / Member / Copan Diagnostics, Inc., United States, Ended: Dec 9, 2025
- Norman Sharples, MSc / Contributor / Copan Diagnostics Inc., United States, Ended: Dec 9, 2025
- Vanessa Tran, PhD, FCCM / Contributor / Public Health Ontario, Canada, Ended: Dec 9, 2025
- Melanie Yarbrough, PhD / Contributor / Washington University School of Medicine, United States, Ended: Jul 13, 2022
- Rodney C. Arcenas, PhD, D(ABMM) / Member / Roche Molecular Systems - Pleasanton, CA, United States, Ended: Nov 13, 2025
- Calvin Ball, MS, MLS(ASCP)cm, CMQ/OE(ASQ), CLS / Contributor / Stanford Health Care - Valley Care/Kaiser Permanente, United States, Ended: Nov 13, 2025
- Laurie Bauer, DO, FCAP, FASCP / Contributor / Louis Stokes Cleveland VA Medical Center, United States, Ended: Nov 13, 2025
- Karen E. Bijwaard, MS, RAC, MB(ASCP) / Contributor / FDA Center for Devices and Radio-logical Health, United States, Ended: Nov 13, 2025
- George Broukhanski, PhD, MS / Member / Public Health Ontario Laboratories, Canada, Ended: Nov 14, 2025
- Julia Bugrysheva, PhD / Contributor / CDC, United States, Ended: Nov 14, 2025
- Davina Campbell, MS, MPH / Contributor / Centers for Disease Control and Prevention, United States, Ended: Nov 13, 2025
- Gerald A. Capraro, PhD, D(ABMM) / Contributor / LabCorp - Burlington, NC, United States, Ended: Nov 13, 2025
- Sugganth Daniel, MD, FCAP / Member / Illumina, United States, Ended: Nov 13, 2025
- James J. Dunn, PhD, D(ABMM), MT(ASCP) / Member / Baylor College of Medicine and Texas Children's Hospital, United States, Ended: Nov 13, 2025
- Helen Fernandes, PhD / Member / Columbia University Medical Center, United States, Ended: Nov 13, 2025
- Lei Fu, PhD, DABCC, FADLM, FCACB / Contributor / Sunnybrook Health Sciences Centre, Canada, Ended: Nov 13, 2025
- Mukesh Gandhi / Contributor / Dynacare (Brampton), Canada, Ended: Nov 13, 2025
- Amy Shirley Gargis, MS, PhD / Member / Centers for Disease Control and Prevention, United States, Ended: Nov 13, 2025
- Alison SL Halpin, PhD / Contributor / Centers for Disease Control and Prevention, United States, Ended: Nov 13, 2025
- Mallory Leetham, MS, MLS(ASCP)cm / Contributor / University of Utah School of Medicine, United States, Ended: Nov 13, 2025
- Mary Beth Minyard, MSCLS, M(ASCP) / Contributor / ResInnova Clinical Diagnostics, United States, Ended: Nov 13, 2025
- Duane W. Newton, PhD, D(ABMM), FIDSA / Member / DWN Consulting, United States, Ended: Nov 13, 2025
- Heddie L. Nichols, PhD, PHM / Contributor / Genentech, United States, Ended: Nov 13, 2025
- Ronald M. Przygodzki, MD / Chairholder / US Department of Veterans Affairs, United States, Ended: Nov 13, 2025
- Honey V. Reddi, PhD, FACMG / Member / Belay Diagnostics, United States, Ended: Nov 13, 2025
- Kate Rhodes, PhD / Member / GRAIL, LLC, United States, Ended: Nov 13, 2025
- Ted E. Schutzbank, PhD, D(ABMM) / Vice-Chairholder / Schutzbank Laboratory Consultants, LLC, United States, Ended: Nov 13, 2025
- Kimberly Starr, PhD, D(ABMM) / Secretary / FirstHealth of the Carolinas Moore Regional Hospital, United States, Ended: Nov 13, 2025
- Nadeem A. Tusneem, MA / Member, United States, Ended: Nov 13, 2025
- J. Rex Astles, PhD, FADLM / Member / Centers for Disease Control and Prevention, United States, Ended: Dec 23, 2025
- Hassan Bayat / Contributor / Sina Clinical Laboratory, Iran, Ended: Dec 23, 2025
- Marvin Berman, PhD / Member / Abbott Laboratories, United States, Ended: Dec 23, 2025
- Bipasa Biswas / Member / FDA Ctr. for Devices/Rad. Health (CDRH), United States, Ended: Dec 23, 2025
- Michelle Renee Campbell, MS, MLS(ASCP)CM, MBCM, SCCM / Member / Mayo Clinic, United States, Ended: Dec 23, 2025
- Karl De Vore, BA, SSBB / Member / Bio-Rad Laboratories, Inc., United States, Ended: Dec 23, 2025
- Ashley Frost, MHA, MLS(ASCP)CM / Member, United States, Ended: Dec 23, 2025
- Kornelia Galior, PhD, DABCC / Contributor / Emory University, United States, Ended: Dec 23, 2025
- Verena Hofmann, PhD / Member / Roche Diagnostics GmbH - Penzberg, GERMANY, Germany, Ended: Dec 23, 2025
- Beimar Iriarte, MS / Member / Abbott Laboratories, United States, Ended: Dec 23, 2025
- Jesper V. Johansen, PhD, MS / Chairholder / Radiometer Medical ApS, Denmark, Ended: Dec 23, 2025
- Paula Ladwig, MS, MLS(ASCP) / Member / Mayo Clinic, United States, Ended: Dec 23, 2025
- Marianne Thuy Duong Le, MS / Member / Qufora A/S, Denmark, Ended: Dec 23, 2025
- Edward Ki Yun Leung, PhD, DABCC, FADLM / Member / Children's Hospital Los Angeles, United States, Ended: Dec 23, 2025
- Ying (Katelin) Mao / Contributor / FDA Center for Devices and Radiological Health, United States, Ended: Dec 23, 2025
- Kristen Meier, PhD / Member / Data Fi, LLC, United States, Ended: Dec 23, 2025
- Ronnie Pedersen, PhD / Member / Radiometer America, Inc., United States, Ended: Dec 23, 2025
- Donna Roberts, MS, MT(ASCP) / Contributor / Baylor Scott and White Health, United States, Ended: Dec 23, 2025
- Scott A. Ruetten, PhD / Member / World Health Organization, United States, Ended: Dec 23, 2025
- Vinita Thakur, PhD, FADLM / Member / Eastern Health - Health Sciences Centre, Canada, Ended: Dec 23, 2025
- Christina Wood-Wentz, MSc / Contributor / Mayo Clinic, United States, Ended: Dec 23, 2025
- Ghaith Altawallbeh, MSc, PhD, DABCC / Contributor / Intermountain Central Laboratory, United States
- Sophie Arbefeville, MD / Member / Marshfield Clinic Health System, United States
- J. Rex Astles, PhD, FADLM / Member / Centers for Disease Control and Prevention, United States
- Sucheta Banerjee Kurundkar, PhD, MBA / Member / Clinical Development Services Agency, Translational Health Science & Technology Institute, India
- Alicia Branch, PhD / Contributor / Centers for Disease Control and Prevention, United States
- Scott Cox / Contributor / Yukon-Kuskokwim Delta Regional Hospital, United States
- Kelly Doyle, PhD, DABCC, FADLM / Contributor / ARUP Laboratories/University of Utah, United States
- Christopher Farnsworth, PhD, DABCC, FADLM / Contributor / Washington University in St. Louis, United States
- Kevin Forbes / Member / Abbott Diagnostics, United Kingdom
- Kiyoshi Ichihara / Member / Yamaguchi University, Japan, Ended: Jun 22, 2022
- Jose Jara Aguirre, MD / Member / Children's Hospital National Medical Center, United States
- Tendayi Jubenkanda / Contributor / Biomedical Research and Training Institute (BRTI), Zimbabwe
- Angela Mariani, BS, PhD / Contributor / BD, United States, Ended: Jan 16, 2023
- Mads Nybo, PhD / Member / Odense University Hospital, Denmark
- Vijay Padayachee / Contributor / Vijay Padayachee, South Africa
- Ronnie Pedersen, PhD / Contributor / Radiometer America, Inc., United States
- Christina Pierre, PhD / Member / Penn Medicine Lancaster General Hospital, United States
- Ghazaleh Pourmahram, PhD, MICR / Member / Virtual Clinical & Scientific Consulting LTD, United Kingdom
- Melissa Richard-Greenblatt, PhD, D(ABMM), FCCM, DTM&H / Member / Hospital for Sick Children, Canada
- Sheryl Thiessen, BSMT, MA, RT(CSMLS), MLS(ASCP) / Vice-Chairholder / Provincial Laboratory Medicine Services, Canada
- Joe Wiencek, PhD, DABCC, FADLM / Chairholder / Vanderbilt University School of Medicine, United States
- Lara Yourkin, BS,MBA / Contributor / National Jewish Health, United States
- Susan Ashrafzadeh Kian, M.S. / Contributor / Mayo Clinic, United States
- Mustafa Barbhuiya / Member / Baystate Health, United States
- Liyun Cao / Member / University of Alabama at Birmingham, United States
- Kelly Doyle, PhD, DABCC, FADLM / Contributor / ARUP Laboratories/University of Utah, United States
- Mari Ishak Gabra, MSc, PhD, SC(ASCP)cm / Contributor / Inter Science Institute, United States
- Zahra Khatami, MS, FRCPath / Member / Royal London Hospitals, United Kingdom
- Vathany Kulasingam, PhD, FCACB / Member / University Health Network and University of Toronto, Canada
- Shirley Li, PhD, MD / Contributor / Ohio State University, United States, Ended: Oct 27, 2022
- Christopher Marquez, MD, DABCC / Contributor / Mayo Clinic Florida, United States, Ended: Mar 15, 2023
- Qing H. Meng, MD, PhD, DABCC, FADLM / Member / The University of Texas MD Anderson Cancer Center, United States, Ended: May 9, 2022
- Zehra Ordulu / Contributor / University of Florida, United States
- Boris Pinchuk / Member / Roche Diagnostics GmbH, Germany
- Ganaesh Ramanathan / Contributor / St James University Teaching Hospital, Leeds NHS University Teaching Hospital NHS Trust
- Alina Sofronescu / Member / North Carolina Baptist Hospital, United States, Ended: May 9, 2022
- Lina Souan, B.Sc., M.Sc., Ph.D., HCEL, HCOSP, HCCAC, D(ABMLI) / Contributor / King Hussein Cancer Center, Jordan
- Catharine Margaret Sturgeon, PhD, FRCPath / Chairholder / Edinburgh Royal Infirmary, United Kingdom
- Huub van Rossum, PhD, EuSpLM / Member / Netherlands Cancer Institute, Netherlands
- Richard Y. Wang, DO / Member / Centers for Disease Control and Prevention, United States
- Sedef Yenice, MS, PhD / Member / EFLM - TURKEY, Turkey
- Chin Fung Yeung, SC(ASCPi)CM , MB(ASCPi)CM / Contributor / PHC Medical Diagnostic Center Ltd, Hong Kong, Ended: Jul 21, 2022
- Zhen Zhao, PhD, DABCC, FAACC / Member / New York Presbyterian/Weill Cornell Medical Center
- Tanis Dingle, PhD, D(ABMM), FCCM / Member / Alberta Precision Laboratories - Public Health Laboratory, Canada
- Philippe J. Dufresne, PhD / Chairholder / Institut national de santé publique du Québec, Canada
- Nathan P. Wiederhold, PharmD / Vice-Chairholder / University of Texas Health Science Center at San Antonio, United States
- Philippe J. Dufresne, PhD / Chairholder / Institut national de santé publique du Québec, Canada
- Zoe Freeman Weiss, MD / Member / Tufts Medical Center, United States
- Nathan P. Wiederhold, PharmD / Vice-Chairholder / University of Texas Health Science Center at San Antonio, United States

Share Your Expertise
