You'll receive the latest updates on new standards, guidelines, and educational resources, as well as expert insights to help enhance your laboratory's performance and compliance.
Insights Blog
Explore the latest developments in laboratory standards, guidelines, and best practices. Our expert perspectives and stories provide up-to-date information about what's new in laboratory testing.
All Insights
Filters
{{ sortLabels[option] }}
July 15, 2020
Children are some of the most difficult patients from whom to collect blood sample specimens. Ensuring safety and efficacy when performing blood draws on pediatric patients, while also accounting for their emotional and physical well-being is of utmost importance.
June 16, 2020
Nucleic Acid Testing Validation and Verification
May 14, 2020
Now, more than ever, it’s important to implement a disaster plan for your lab or update your lab’s current plan. Emergencies can impede your lab’s efficiency and sometimes even bring operations to a standstill. Preparation can help ensure that your lab continues to deliver high quality results and keep patients and laboratory workers healthy during the COVID-19 pandemic.
Antimicrobial Susceptibility Testing
Microbiology
April 27, 2020
We spoke with Ted Schutzbank, PhD D(ABMM), Scientific Affairs Manager of Diagnostics at Meridian Bioscience about the importance of standardization in molecular diagnostics and the new testing methods available for COVID-19.
April 10, 2020
Written By Sherry A. Dunbar, PhD, MBA, Senior Director, Global Scientific Affairs, Luminex Corporation and Shubhagata Das, Scientific Affairs Manager, Luminex Corporation
As of April 8, 2020, the...
Public Health (1)
March 30, 2020
Performing a Biological Risk Assessment in the Laboratory
March 24, 2020
March 9, 2020
Taking blood samples from children can be difficult for the phlebotomist. Ensuring safety while accounting for a pediatric patient’s emotional and physical well-being is of the utmost importance.
February 28, 2020
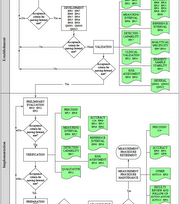
Measurement procedure comparison is one of the most common techniques used by both manufacturers and medical laboratorians to estimate the bias of an in vitro diagnostic (IVD) measurement procedure relative to a comparator.
No Results
No results were returned for that query.