AST News Update June 2023: The Latest on Testing Cefiderocol
6/16/2023
Andrea Ferrell, BD Life Sciences, Sparks, Maryland, USA
Janet Hindler, Los Angeles County Department of Public Health, Los Angeles, California, USA
In the July 2020 issue of this News Update, cefiderocol (trade name Fetroja®, manufactured by Shionogi) was discussed to include its projected use, requirements for testing, and more. Since that time, several new developments have occurred for cefiderocol testing. This article will bring clinical laboratorians up to date with key facts about testing cefiderocol and a recent warning statement that was added to CLSI M100-Ed33.
In 2020, CLSI cefiderocol breakpoints were considered investigational (INV) because cefiderocol was not yet approved by the US Food and Drug Administration (FDA) for clinical use. FDA has since approved cefiderocol for treatment of adults with complicated urinary tract infections (cUTIs) and hospital-acquired and ventilator-associated bacterial pneumonia due to Pseudomonas aeruginosa, Acinetobacter baumannii complex (pneumonia only), and several members of the Enterobacterales as described here.
Key facts about in vitro testing of cefiderocol:
1. Cefiderocol is considered a last resort drug for treatment of infections due to multidrug resistant gram-negative aerobic bacteria1,2 and is typically tested and reported on these bacteria only.
2. Cefiderocol has no clinically relevant in vitro activity against most gram-positive bacteria or anaerobic bacteria, and there are no recommendations for testing these organism groups.
3. Broth dilution testing of cefiderocol requires the use of iron-depleted, cation-adjusted Mueller-Hinton broth.
4. Disk diffusion testing of cefiderocol is performed on routine Mueller-Hinton agar. There is no need to obtain special iron-depleted Mueller-Hinton agar for disk diffusion testing.
5. FDA-cleared cefiderocol disks are available from three manufacturers (see Table 1).
6. For manual broth microdilution, two FDA-cleared assays are available (ComASP and Sensititre), in addition to a Sensititre research use only (RUO) test (see Table 1). An RUO gradient strip from Liofilchem is available for testing P. aeruginosa only (see Table 1).
7. Currently, cefiderocol is not available on any automated AST system.
8. Isolates for verification or validation of cefiderocol tests are available from:
• CDC & FDA Antibiotic Resistance (AR) Isolate Bank
• Laboratory Specialists, Inc. (LSI)
9. LSI provides reference broth microdilution minimal inhibitory concentration (MIC) testing of cefiderocol and is a CLIA-certified laboratory.
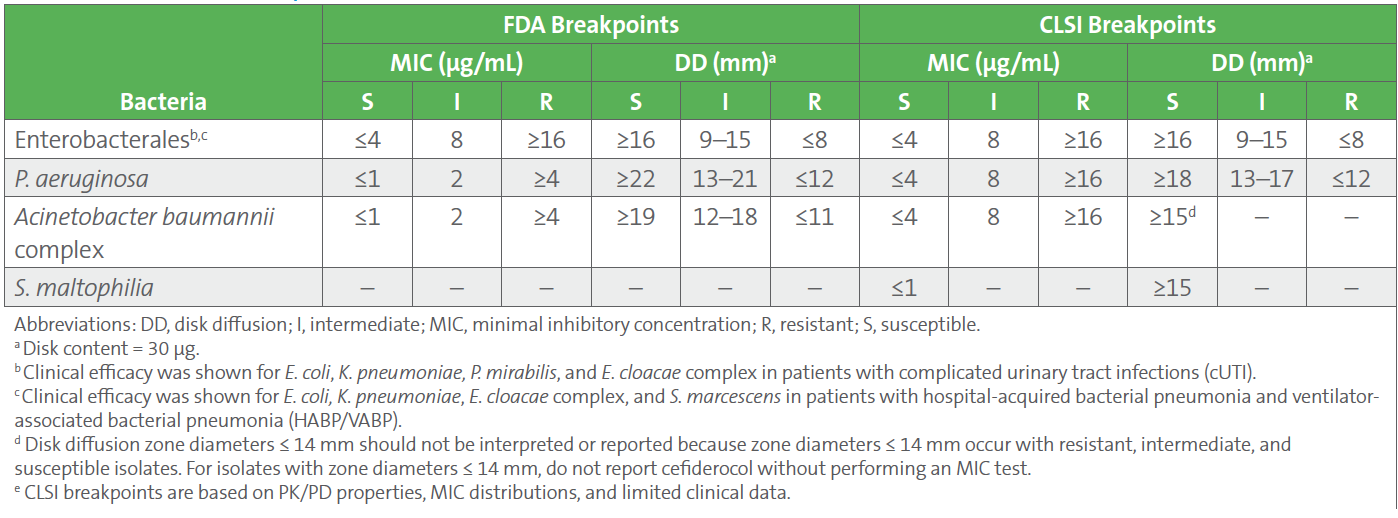
10. Breakpoints for cefiderocol provided by CLSI and FDA differ for P. aeruginosa and A. baumannii complex (see Table 2). FDA-cleared tests are based on FDA breakpoints, and use of alternative breakpoints for FDA-cleared tests require validation. EUCAST has cefiderocol breakpoints only for Enterobacterales and P. aeruginosa; the breakpoints can be found here.
Shionogi maintains an up-to-date listing of available testing methods here.
Table 1. Testing Options for Cefiderocol in Addition to Reference Broth Microdilution
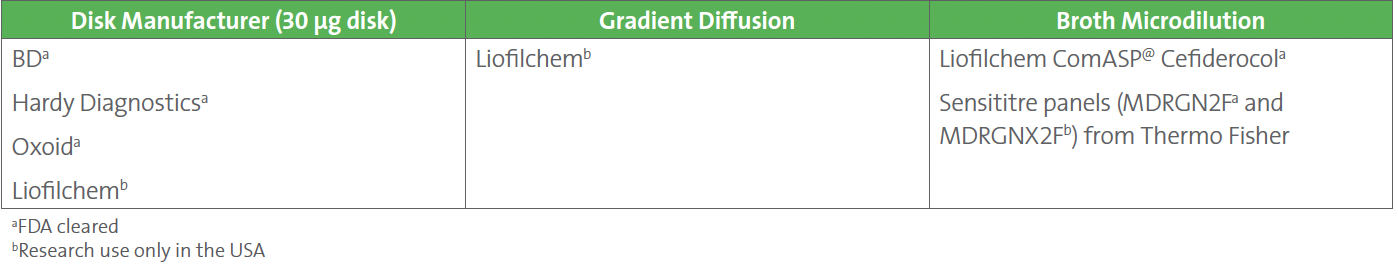
Table 2. FDA and CLSI Breakpoints for Cefiderocol3,4
There are challenges with reading results from both disk diffusion and reference broth microdilution MIC testing of cefiderocol. These were addressed early after the drug achieved FDA approval, in a minireview by Simner and Patel,5 and broth microdilution endpoints are also shown in CLSI M100 Appendix I.3 Since that time, additional testing issues have come to light, leading CLSI to add the following statement to the 2023 edition of CLSI M100, to emphasize these challenges: “The accuracy and reproducibility of cefiderocol testing results by disk diffusion and broth microdilution are markedly impacted by iron concentration, inoculum preparation, and may vary by disk and media manufacturer. Depending on the type of variance observed, false resistant or false susceptible results may occur. Testing subsequent isolates is encouraged. Discussion with prescribers and antimicrobial stewardship members about the potential for inaccuracies is recommended.” Members of the CLSI AST Subcommittee have provided additional guidance to support this statement.6
References
1 Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America antimicrobial-resistant treatment guidance: Gram-negative bacterial infections. Infectious Diseases Society of America 2022; Version 1.1. Available at www.idsociety.org/practice-guideline/amr-guidance/. Accessed June 1, 2023.
2 Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America Guidance on the treatment of AmpC β-lactamase-producing Enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Infectious Diseases Society of America 2022; Version 2.0. Available at www.idsociety.org/practice-guideline/amr-guidance-2.0/. Accessed June 1, 2023.
3 CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 33rd ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2023. Access here.
4 US Food and Drug Administration. Antibacterial Susceptibility Test Interpretive Criteria (STIC). https://www.fda.gov/drugs/development-resources/antibacterial-susceptibility-test-interpretive-criteria, 2023. Accessed June 1, 2023
5 Simner PJ, Patel R. Cefiderocol antimicrobial susceptibility testing considerations: the Achilles’ heel of the Trojan horse? J Clin Microbiol. 2020;59(1). DOI: https://doi.org/10.1128/JCM.00951-20.
6 Simner PJ, Palavecino EL, Satlin MJ, et al. Potential of inaccurate cefiderocol susceptibility results: a CLSI AST subcommittee advisory. J Clin Microbiol. 2023. 61:e0160022. doi: 10.1128/jcm.01600-22.
